When prayers and efforts come together, humans can fight and win against all odds.
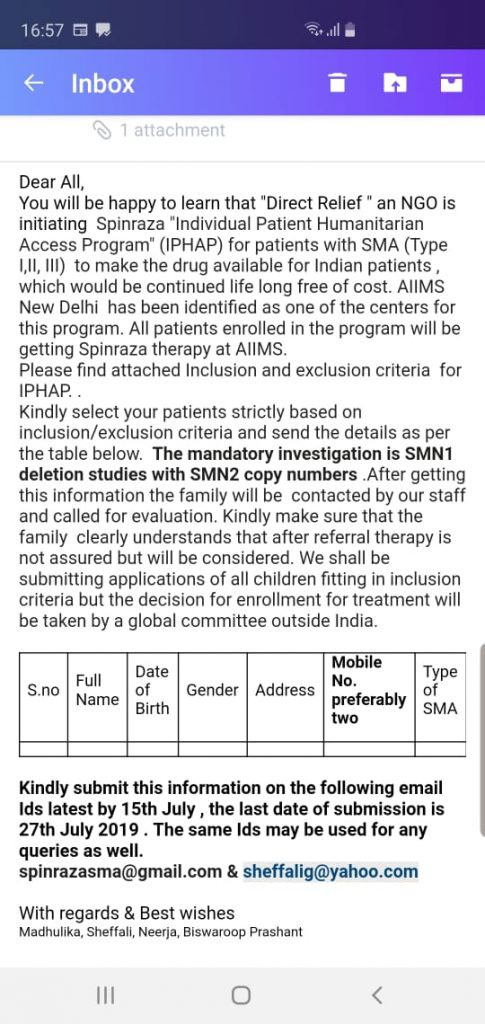
CureSMA India is pleased to inform that “Direct Relief”, a US based NGO has initiated SPINRAZA “Individual Patient Humanitarian Access Program” (SIPHAP) in coordination with CureSMA India for patients with SMA (Type I, II, III) to make the drug available for Indian patients.
This program will be available for limited number of eligible patients with SMA (Type I, II, III). The last date is 30th July, 2019. The applications have to go through one of the four centers allocated for this as below:
1. All India Institute of medical Science, New Delhi
2. Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow
3. KEM Hospital, Mumbai
4. Baptist Hospital, Bangalore.
Please follow the instructions/steps mentioned below while submitting the application:
Step 1 – Application: Patients to approach their Doctor (Pediatrician, Clinicians OR Neurologist) and share about this program. Doctor will then send patient’s data (Name, Address, DOB, Contact numbers (preferably two), SMA type) along with copy of SMA1 gene test report to spinrazasma@gmail.com and/or sheffalig@yahoo.com (AIIMS, Delhi), kempediatric19sma@gamil.com (KEM Hospital, Mumbai), shubharaophadke@gmail.com (SGPGIMS, Lucknow), Rare Disease Helpline- 8892555000 (Baptist Hospital, Bangalore). Patients may contact any of CureSMA India Core Team Member for more details.
Step 2 – Assessment & Evaluation: Patients to approach one of the four approved centers/hospitals (as shared in the images) whichever is convenient to patient for complete evaluation along with the required documents/reports (SMN1 test report, SMN2 copies number report, full spine x-ray, CBC and Liver Function test reports, if possible. These tests can be performed in the allocated four centers as well. A team of Doctors will carry out the assessment & evaluation based on Inclusion and Exclusion Criteria provided by Direct Relief. The evaluation data of eligible patients will then be finalized by striking the name of patient and allocating unique number to patient. Finalized data will then be uploaded in Direct Relief Portal by respective Doctors from the four approved centers. The last date for uploading all the data by Doctors on Direct Relief Portal is 30th July 2019.
For patients in South India, five evaluation centers have been arranged for the convenience of patients. They are in Hyderabad, Chennai, Trivandrum, Kozhikode and Calicut. The assigned Doctors in evaluation centers will send Patient’s evaluation data to Baptist Hospital, Bangalore and data of eligible patients will be finalized by striking the name of patient and allocating unique number to patient.
Finalized data will then be uploaded to Direct Relief Portal by Doctors in Baptist Hospital. The last date for uploading all the data by Doctors on Direct Relief Portal is 30th July 2019.
Step 3 – The submitted eligible patient data uploaded in Direct Relief portal will then be studied by global team of Doctors in USA by means of a blind screening which means that the Doctors will only see patient unique number and parameters. This team will take the final call on most suitable 25-30 patients.
Step 4 – Direct Relief will then arrange delivery of medicine to India for selected 25-30 patients. Selected patients will be administered the medicine in any of the four approved centers based on convenience of the patient. The entire process may take around 3-4 months and is expected to begin around Oct 2019. It is to be noted that this is the FIRST BATCH of 25-30 patients.
Note: This is a pilot program and an Independent Global Medical Expert Committee (MEC) of SMA specialists will decide the limited number of patients (25-30) depending upon the exclusion and inclusion criteria. CureSMA India is hoping for the success of this program which will result in more of such programs in the coming course of time and many more SMA patients are expected to get the life saving medicine and get a chance to live a healthy life.
We can only ensure that each and every patient registered with us gets the fair chance of applying for spinraza based on criteria decided by Direct Relief and the team of Doctors in four centers.
Disclaimer: CureSMA India or any doctor cannot influence the decision of Independent Global Medical Expert Committee (MEC) of SMA specialists.